Atomic Structure (Bohr Model) for Beryllium (Be) YouTube
Electron Configuration for Beryllium (Be, Be2+ ion)
The model shown in the figure below is for the beryllium atom or any four‐electron ion. Note that the occupancy of the inner orbit is restricted to two electrons (Pauli Principle) and that the orbit radii are constrained by the hydrogen atom result (R 2 = 4R 1 ) leaving only one variational parameter, the radius of the n = 1 orbit.
ShowMe beryllium bohr model
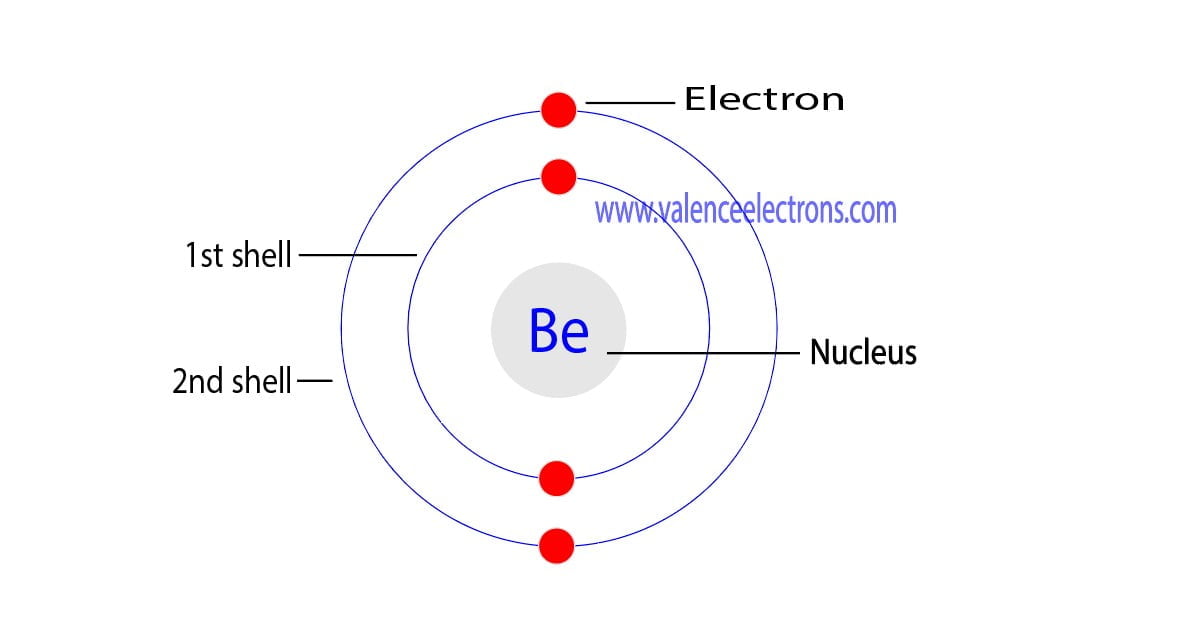
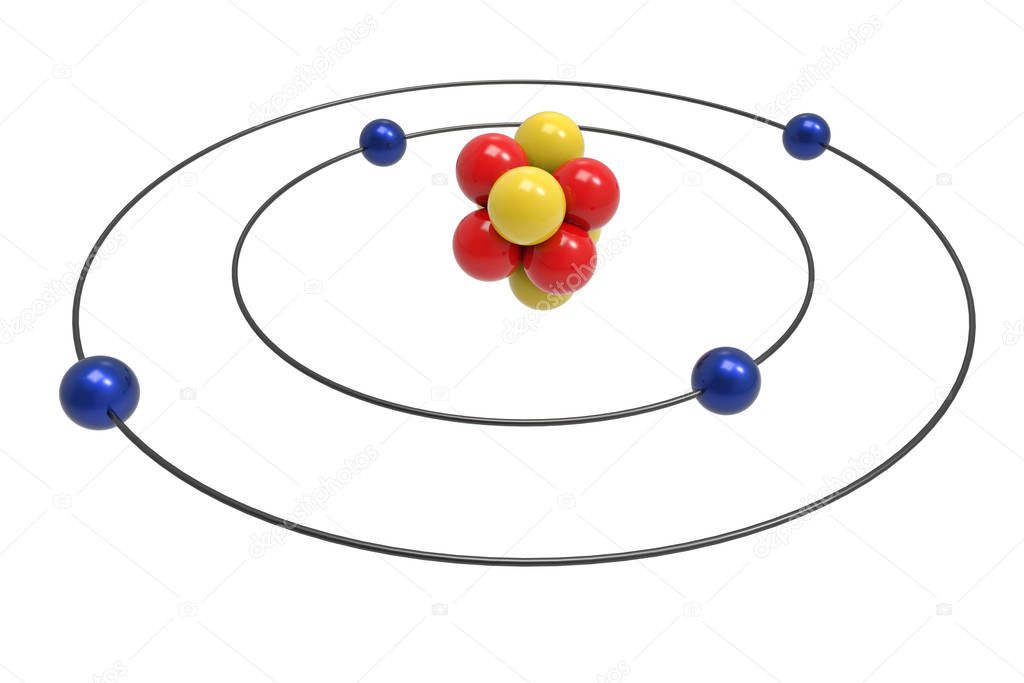
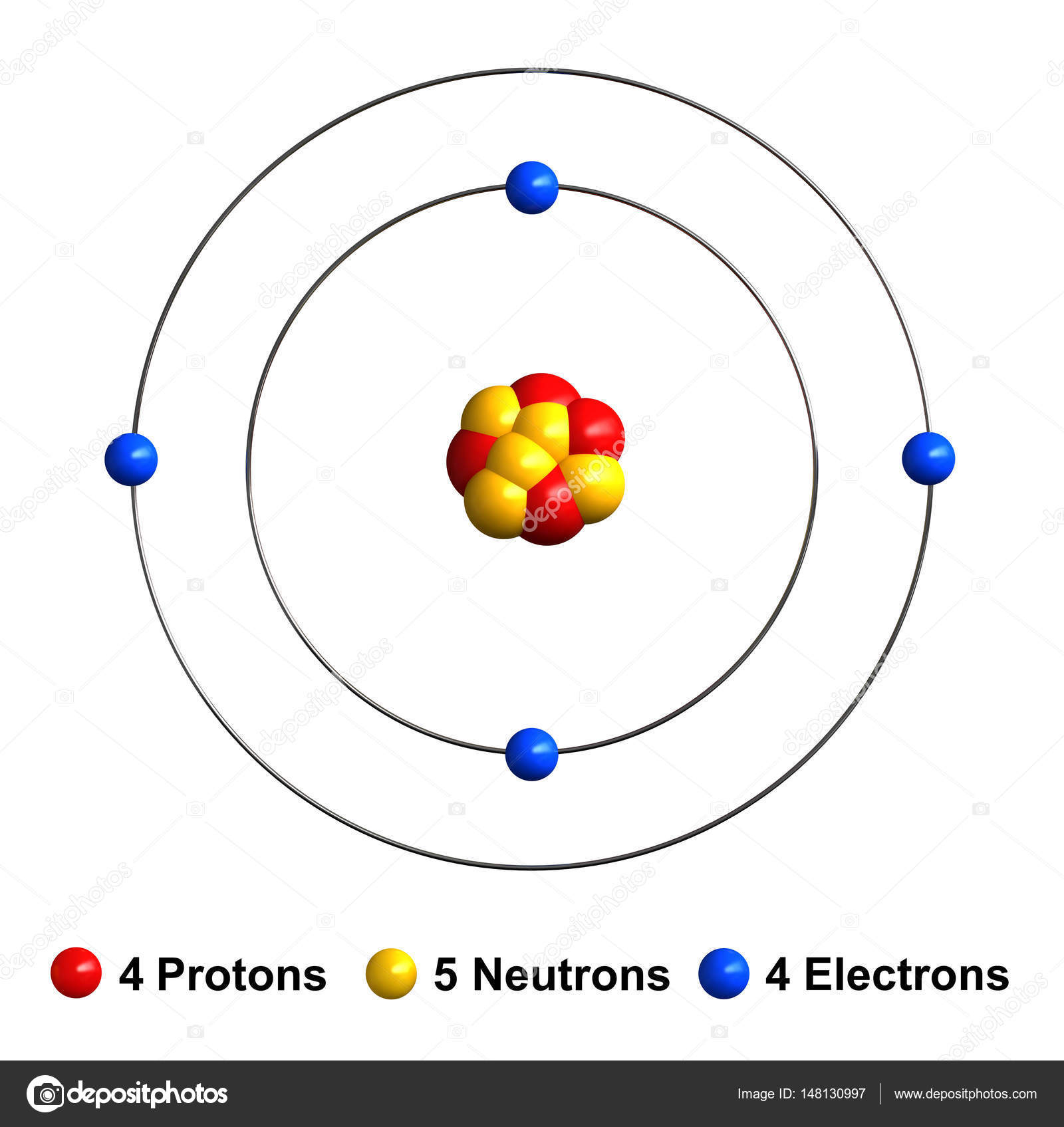
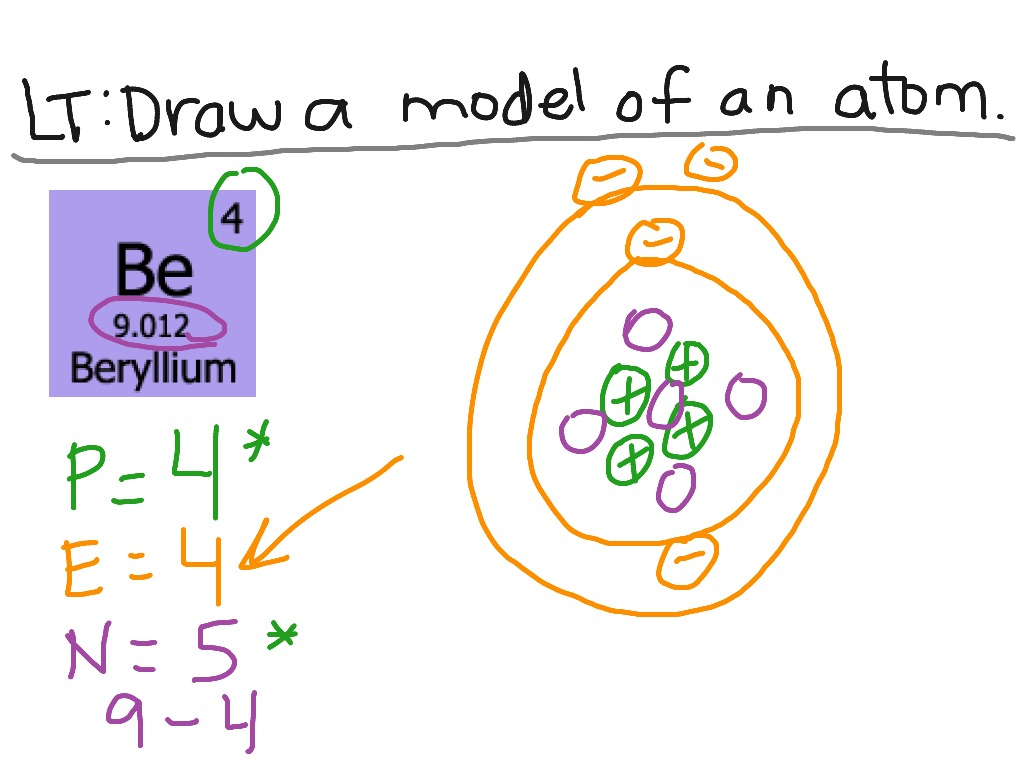
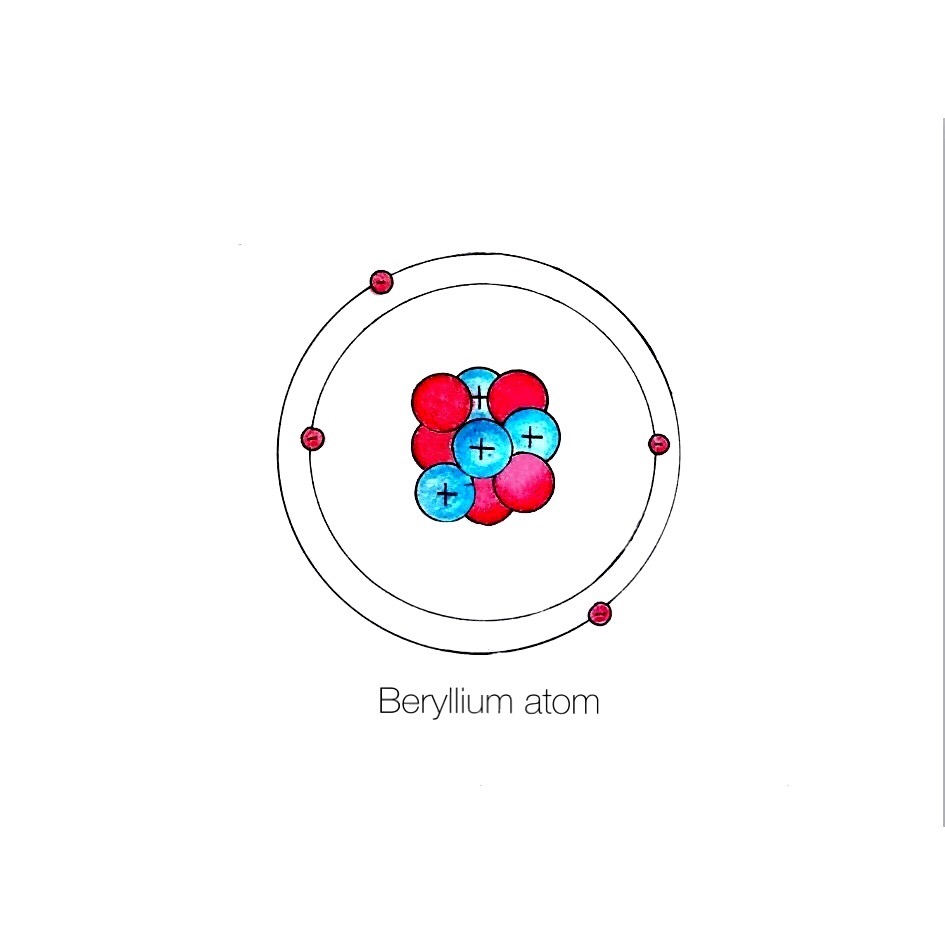
The Bohr Model of Beryllium (Be) has a nucleus that contains 5 neutrons and 4 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Beryllium contains 2 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Beryllium (Be)?

Beryllium Bohr Diagram Transborder Media
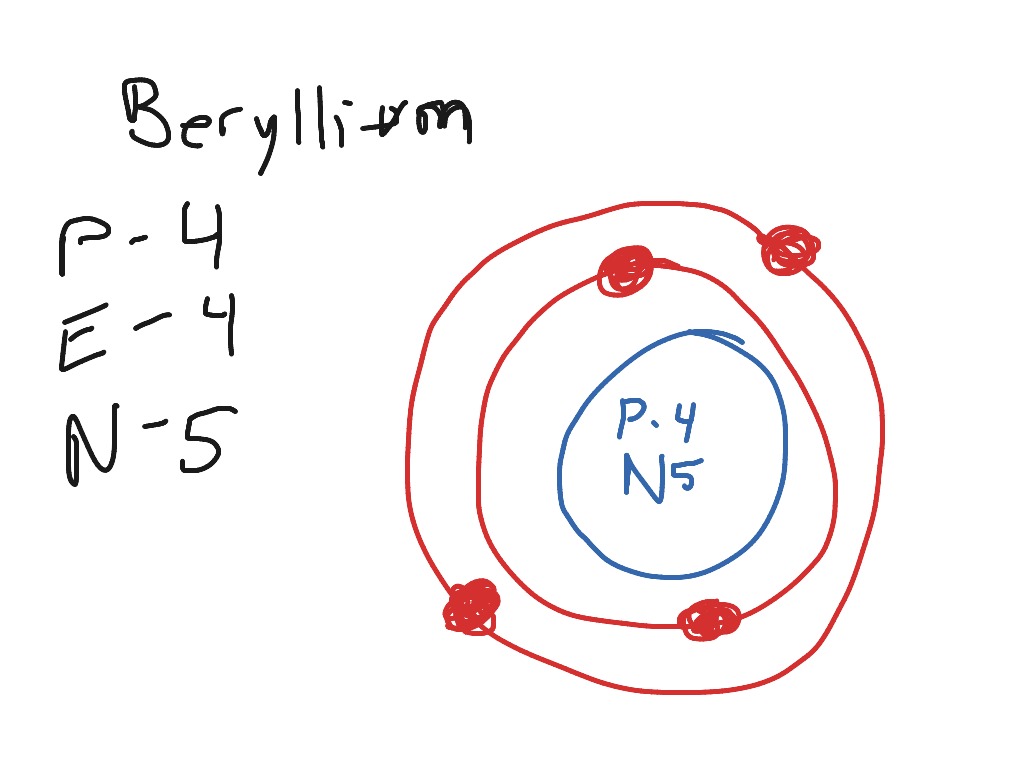
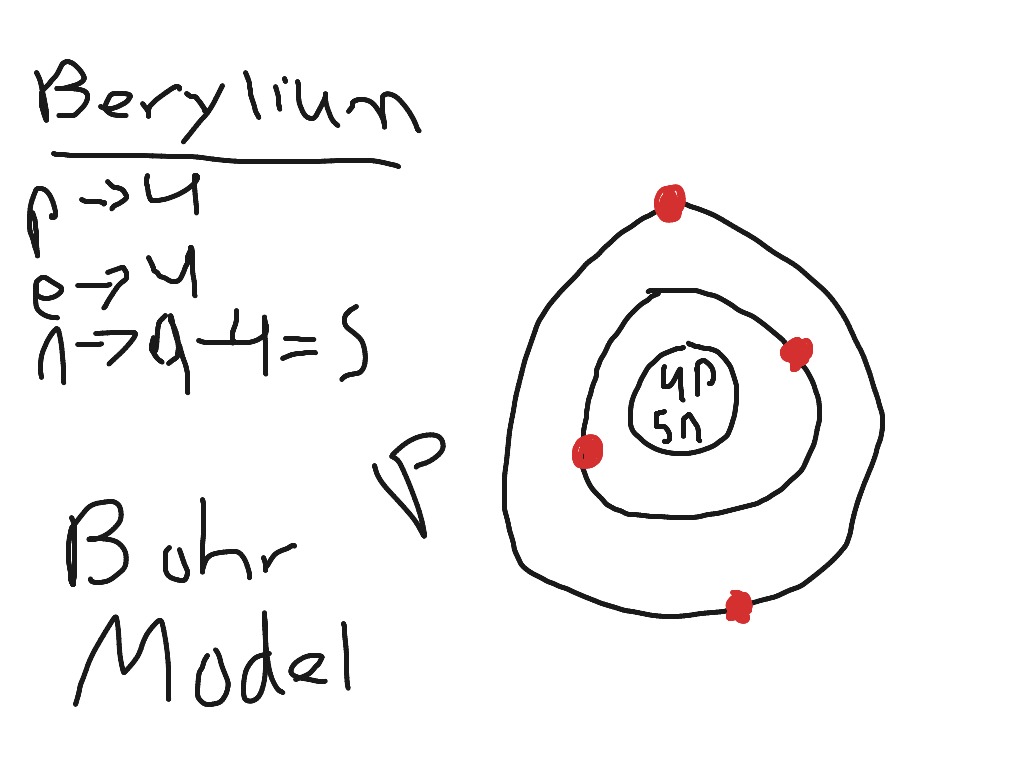
The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. The Mass number is 9 which means Beryllium needs 5 neutrons in the nucleus. (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons.
Beryllium model Stock Vector Images Alamy
Beryllium is the 4th element in the periodic table.. (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Beryllium atom electron configuration through orbit.
Beryllium Bohr Diagram
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.

Atomic Structure (Bohr Model) for Beryllium (Be) YouTube
Bohr model of Elements. 1. Hydrogen (H) 1. 2. Helium (He) 2. 3. Lithium (Li)

Beryllium Bohr Diagram
34 4.8K views 1 year ago In this video we'll look at the atomic structure and Bohr model for the Beryllium atom (Be). We'll use a Bohr diagram to visually represent where the electrons are.
Modelo Bohr de Átomo de Berilio con protones, neutrones y electrones
6.2 The Bohr Model; 6.3 Development of Quantum Theory; 6.4 Electronic Structure of Atoms (Electron Configurations) 6.5 Periodic Variations in Element Properties;. the beryllium and boron atoms each have only four and six electrons, respectively. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in.

Bohr Diagram For Beryllium General Wiring Diagram
Atomic & Molecular Structure How to Make a 3-D Bohr Model ••• Updated April 24, 2017 By Tricia Lobo In your introductory chemistry classes you will have to become familiar with a number of the early models of atoms, which represent scientists' early concepts of the structure of atoms.
Beryllium Bohr Diagram exatin.info
Ai = hν = hc λ A i = h ν = h c λ. Unknown wavelength λ λ can be determined from the Rydberg formula: 1 λ =R∞Z2( 1 n21 − 1 n22) 1 λ = R ∞ Z 2 ( 1 n 1 2 − 1 n 2 2) so that final equation for ionization energy looks like this: Ei = hc e R∞Z2( 1 n21 − 1 n22) E i = h c e R ∞ Z 2 ( 1 n 1 2 − 1 n 2 2)
Labelled Diagram Of An Atom Of Beryllium Labeled Diagram
Bohr model of sodium atom; beryllium atom Bohr model; In 1913, Danish physicist Neil Bohr proposed the Bohr atomic model based on Planck's quantum theory of radiation. This atomic model is the modification of Rutherford's atomic model (the nucleus is positively charged and is surrounded by electrons (negatively charged particles).
Beryllium Bohr Diagram exatin.info
Welcome to Chem How, in this video atomic structure of Beryllium is shown.This atomic model is according to Bohr's model of atoms.According to Bohr's model o.
Bohr Diagram For Beryllium General Wiring Diagram
enhance student understanding of atomic structure. A Bohr model, more correctly a deBroglie-Bohr model, is used here to calculate the total electronic energies of atoms and ions containing up to four electrons. The Bohr model for the hydrogen atom is the prototype of the semi-classical approach to atomic and molecular structure.
Periodic Table Beryllium Protons Neutrons Electrons Periodic Table
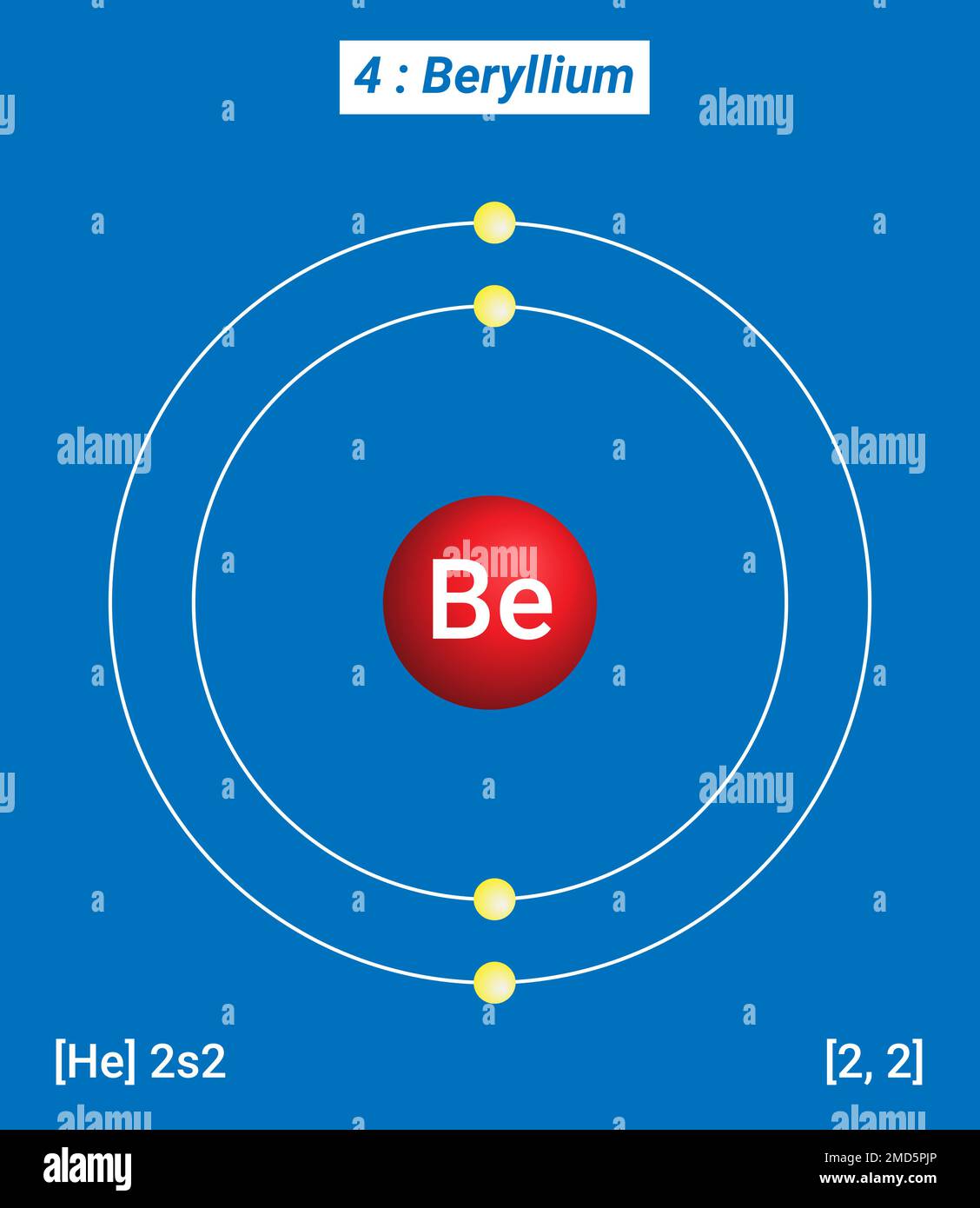
Electrons arrangement or Bohr model: 2, 2: Electronic configuration [He] 2s 2: Atomic radius: 153 picometers (van der Waals radius) Valence electrons: 2: 1st Ionization energy: 9.323 eV:. You have already seen the Bohr model of Beryllium atom in the above table and you have seen that the number of orbits or shells in sodium is 2.

Beryllium Bohr Model
The calculation for the beryllium atom is carried out as shown below. Required student input is indicated by the highlighted regions. Enter nuclear charge: Z := 4 Kinetic energy: T1 ( R1 ) 1 := 2 ⋅ R12 1 T2 ( R1 ) := 8 ⋅ R12 Electron‐nucleus potential energy: − Z VN1 ( R1 ) := R1 − Z VN2 ( R1 ) := 4 ⋅ R1

Bohr Diagram For Beryllium
The Bohr model explains the stability of the atom and atomic particles. It also talks about the position of various atomic particles inside the atom as well as their charge and other properties. It describes the structure of the atom in detail.