How to Balance NaOH + H2SO4 = Na2SO4 + H2O YouTube
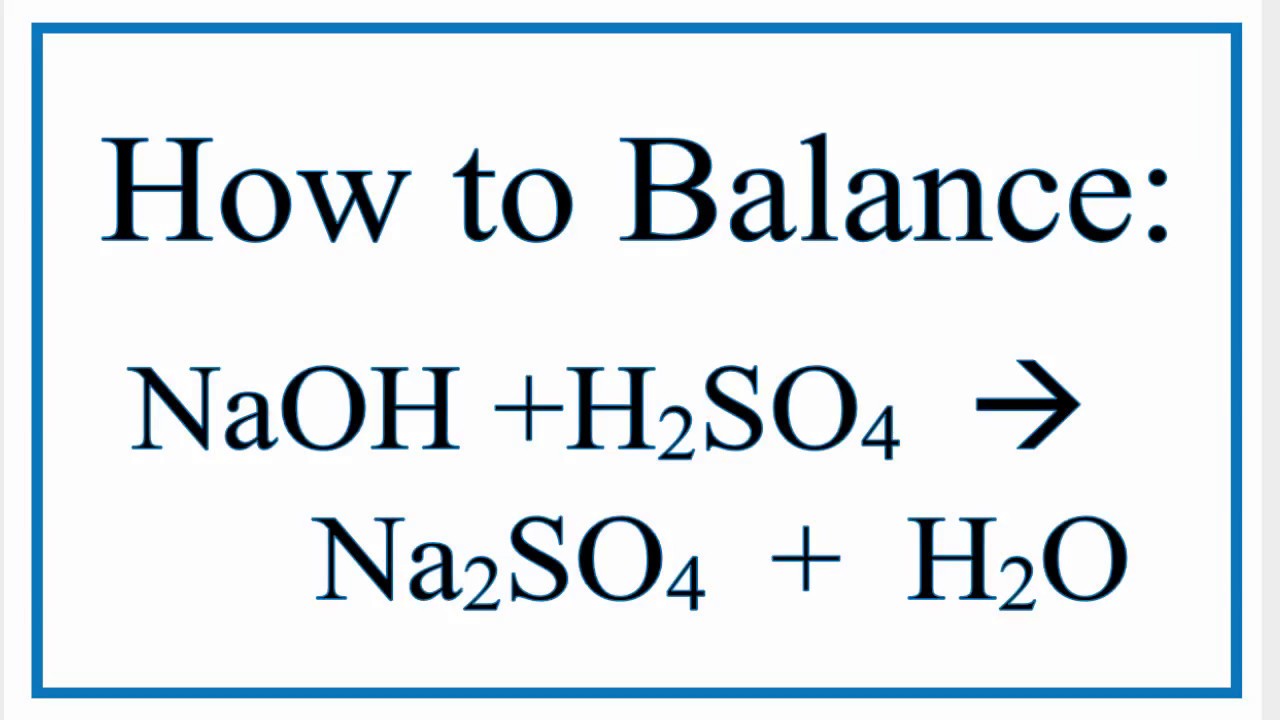
How to Balance NaOH + H2SO4 = Na2SO4 + H2O YouTube
Instant Answer: Step 1/2 First, we write the balanced molecular equation for the reaction between sodium hydroxide (NaOH) and sulfuric acid (H2SO4). NaOH (aq) + H2SO4 (aq) → Na2SO4 (aq) + 2H2O (l) This equation is balanced as there are equal numbers of each type of atom on both sides of the equation. Answer

Balanced Equation For Sodium Hydroxide And Sulfuric Acid
To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored.

Balance the following equation step wise NaoH+H2so4Na2so4+h2o
There are three main steps for writing the net ionic equation for H2SO4 + NaOH = Na2SO4 + H2O (Sulfuric acid + Sodium hydroxide). First, we balance the molec.

Balance the NaOH + H2SO4 Na2SO4 + H₂Oequation Brainly.in
Balanced Chemical Equation 2 NaOH + H 2 SO 4 → Na 2 SO 4 + 2 H 2 O ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Word Equation Sodium Hydroxide + Sulfuric Acid = Sodium Sulfate + Water

step by step balanced equation NaOH+H2SO4=Na2SO4+H2O Brainly.in
To balance NaOH + H2SO4 = Na2SO4 + H2O you'll need to watch out for two things. First, be sure to count all of H, Na, S, and O atoms on each side of the chemical equation..more.more.

How to balance NaOH + H2SO4 = Na2SO4 + H2O Chemical equation
Balanced Chemical Equation H 2 SO 4 + 2 NaOH → Na 2 SO 4 + 2 H 2 O ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Word Equation Sulfuric Acid + Sodium Hydroxide = Sodium Sulfate + Water

H2SO4 + NaOH Sulfuric acid(H2SO4) and Sodium hydroxide(NaOH)What is
Balancing step by step using the inspection method Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 NaOH + 1 H 2 SO 4 = 1 Na 2 SO 4 + 1 H 2 O For each element, we check if the number of atoms is balanced on both sides of the equation. Na is not balanced: 1 atom in reagents and 2 atoms in products.

Identify the acidconjugate base pair in this balanced equation H2SO4
Write a balanced neutralization equation for the reaction of calcium hydroxide with sulfuric acid This page titled 8.4: Acids-Bases Reactions: Neutralization is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young ( ChemistryOnline.com ) via source content that was edited to the style and standards of.
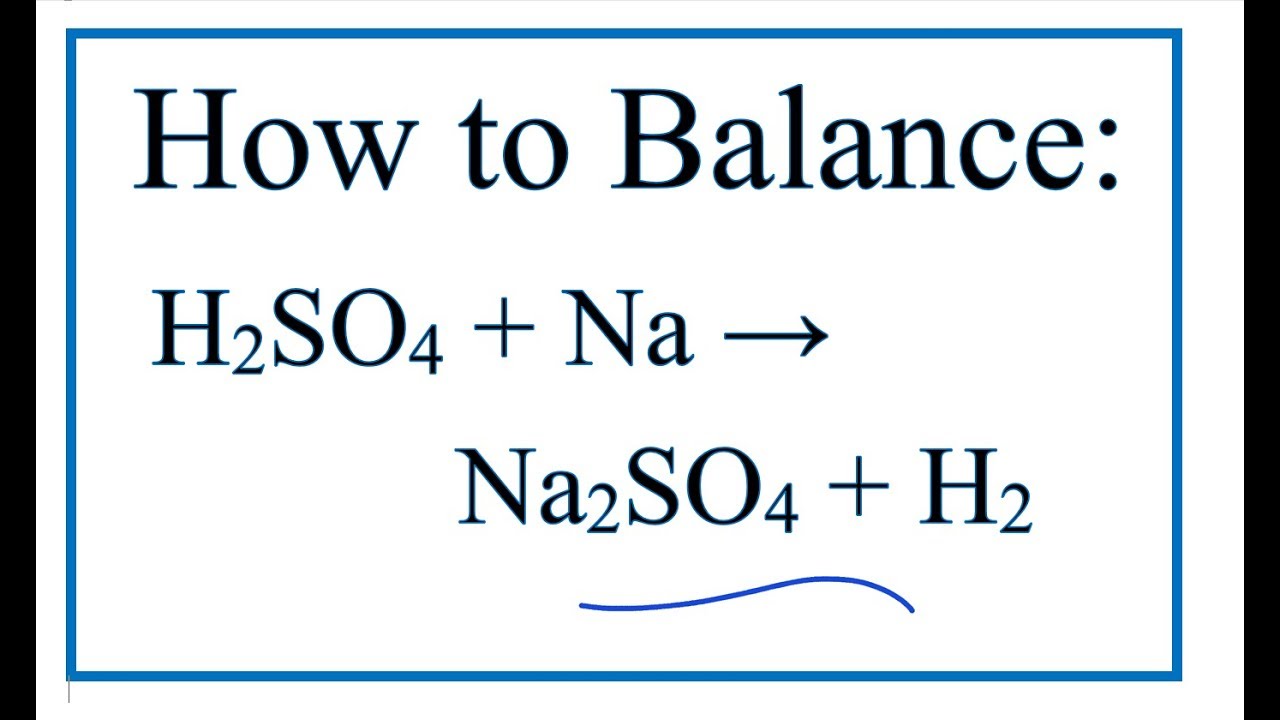
How to Balance H2SO4 + Na = Na2SO4 + H2 (Sulfuric acid + Sodium) YouTube
To balance the chemical equation NaOH + H2SO4 = Na2SO4 + H2O you first must correctly count all of atoms on each side of the chemical equation. Almost yours: 2 weeks, on us 100+ live.

H2so4 Naoh Balanced Equation AdelaideewaWood
Transition Metals and Coordination Compounds 2h 7m. Complete and balance each acid-base equation. c. H2SO4 (aq) + NaOH (aq)¡.

Easy Tips To Balance Naoh H2so4 Na2so4 H2o Youtube
Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. Example: H 2 + O 2 = H 2 O. Count the number of H and O atoms on both sides. There are 2 H atoms on the left and 2 H atom on the right.

Easy tips to balance NaOH + H2SO4 = Na2SO4 + H2O YouTube
The reaction is as follows: NaOH + H2SO4 → Na2SO4 + H2O Sodium Sulphuric Sodium Water Hydroxide Acid Sulphate The reactant is Sodium Hydroxide and Sulphuric acid with the chemical formula NaOH and H2SO4. This is an acid-Base reaction which is called a neutralization reaction and the formation of salt as a product takes place.

How to balance NaOH + H2SO4→ Na2SO4+ H2O YouTube
Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of NaOH + H2SO4 = NaHSO4 + H2O, the equation is balanced. Balance the reaction of NaOH + H2SO4 = NaHSO4 + H2O.

Equation for NaOH + H2O (Sodium hydroxide + Water) YouTube
Balanced Chemical Equation - Solution The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of the original atoms.

How to Write the Net Ionic Equation for H2SO4 + NaOH = Na2SO4 + H2O
1. Firstly, clean the burette with distilled water (DW), and then rinse it with sodium hydroxide (NaOH) solution. This ensures that the interior portion of the burette is coated with a thin layer of NaOH solution. 2. Clean the pipette with DW, and rinse it with dilute solution of H 2 SO 4.

OMTEX CLASSES Balance the following equation stepwise NaOH(aq
The balanced chemical equation is as follow: 2N aOH +H 2SO4 → N a2SO4 +2H 2O. Was this answer helpful? 65. Similar Questions. Q 1. Balance the following equations. a)Pb (NO 3) 2 gives PbO +NO 2 +O 2. b)NH 3 +CuO gives Cu+N 2 +H 2 O.