
Vraylar Approved as Adjunctive Therapy for Major Depressive Disorder
VRAYLAR is an oral, once-daily atypical antipsychotic approved for the acute treatment of adults with manic or mixed episodes associated with bipolar I disorder (3 to 6 mg/day) and for the.
DailyMed VRAYLAR cariprazine capsule, gelatin coated VRAYLAR
U.S. FDA Approves VRAYLAR ® (cariprazine) as an Adjunctive Treatment for Major Depressive Disorder. Approval marks fourth indication for VRAYLAR, backed by proven efficacy and well-established tolerability as an adjunctive treatment for major depressive disorder (MDD) with an antidepressant therapy (ADT), showing improvement in symptoms when compared to placebo + ADT

Cariprazine FDAApproved as Adjunctive Therapy to Antidepressants
19/12/2022. Richter Gedeon's ('Richter') partner AbbVie ('AbbVie') today announced that the U.S. Food and Drug Administration (FDA) has approved VRAYLAR® (cariprazine) as an adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD) in adults. Supported by clinical data demonstrating efficacy and well.
Allergan and Gedeon Richter Receive U.S. FDA Approval
The FDA has approved cariprazine (Vraylar; AbbVie, Gedeon Richter) as an adjunctive therapy to antidepressants for treating adult patients with major depressive disorder (MDD). Cariprazine is administered at a starting dosage of 1.5 mg once daily, which can be increased to 3 mg once daily on day 15, depending on clinical response and tolerability.

Vraylar FDA prescribing information, side effects and uses
Cariprazine is now FDA-approved as an adjunctive therapy to antidepressants. The US Food and Drug Administration (FDA) has approved AbbVie's cariprazine (Vraylar) as an adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD) in adults. 1. "Many living with [MDD] find that their ongoing antidepressant.

Vraylar (cariprazine) for the Treatment of Bipolar Disorder and
VRAYLAR is an oral, once-daily atypical antipsychotic approved for the acute treatment of adults with manic or mixed episodes associated with bipolar I disorder (3 to 6 mg/day) and for the.
BUY Cariprazine (Vraylar) 1.5 mg/1 from GNH India at the best price
Zacks Equity Research. AbbVie ABBV announced that the FDA has approved Vraylar (cariprazine) for the adjunctive treatment of patients with major depressive disorder (MDD). Vraylar is presently.

Allergan and Gedeon Richter Receive U.S. FDA Approval
- Approval marks fourth indication for VRAYLAR, backed by proven efficacy and well-established tolerability as an adjunctive treatment for major.

重度抑郁症新疗法:艾伯维Cariprazine辅助治疗在3期研究中达到主要终点海鸥药房网
December 19, 2022. The Food and Drug Administration (FDA) has approved Vraylar ® (cariprazine) as adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD) in.

Vraylar (cariprazine) for the Treatment of Bipolar Disorder and
NORTH CHICAGO, Ill. , Dec. 16, 2022 /PRNewswire/ -- ABBV ie (NYSE: ABBV) today announced that the U.S. Food and Drug Administration (FDA) has approved VRAYLAR ® (cariprazine) as an adjunctive.
U.S. FDA Approves VRAYLAR® (cariprazine) as an Adjunctive Treatment for
The indication expands the marketed use of the atypical antipsychotic drug to be used with antidepressants in adult patients. The US Food and Drug Administration (FDA) has approved cariprazine (VRAYLAR) as an adjunctive treatment with antidepressant therapy for major depressive disorder. The approval, granted to AbbVie on Friday, is the latest.
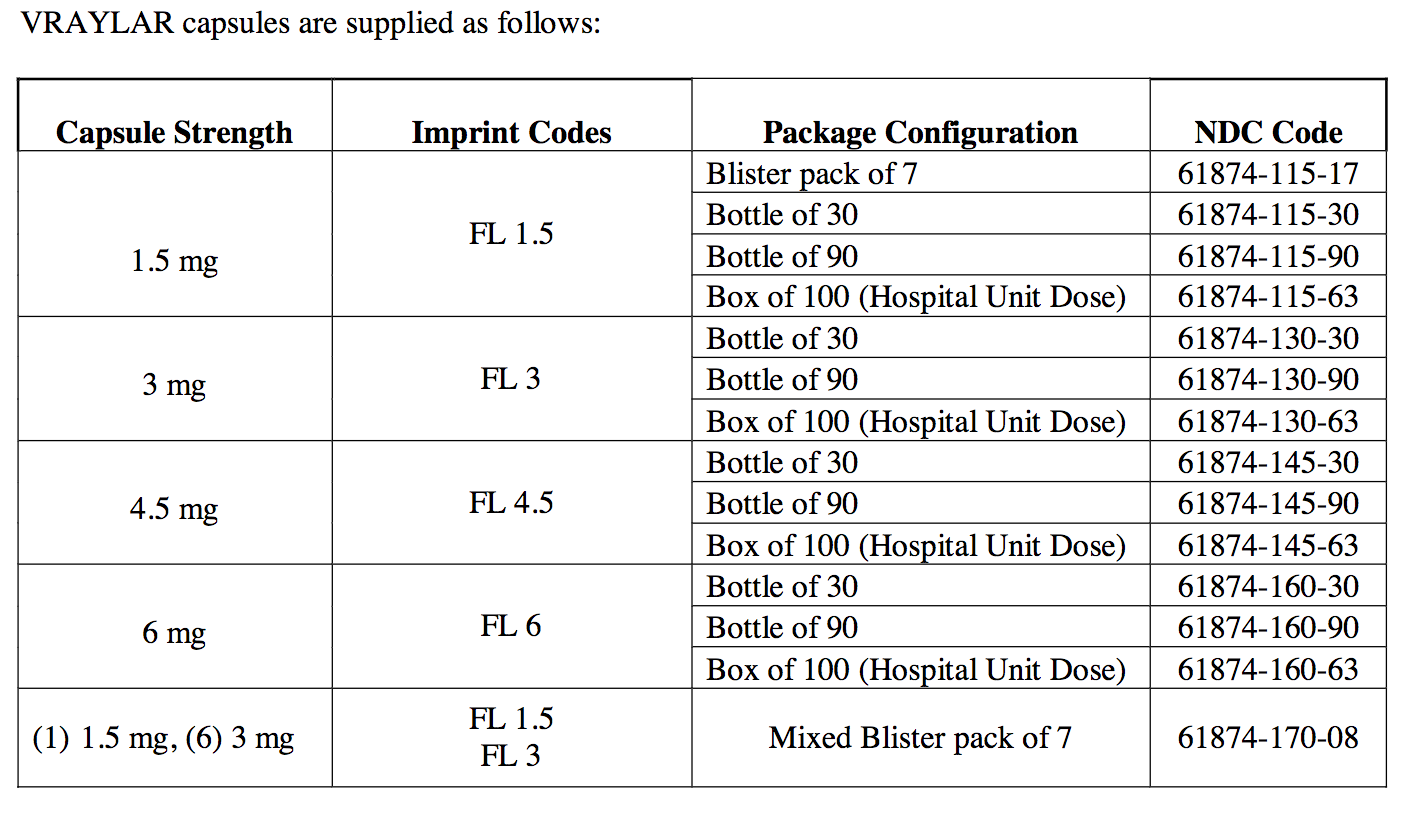
Cariprazine (vraylar) Full Prescribing Information HealthyPlace
Date Article; Dec 16, 2022: Approval U.S. FDA Approves Vraylar (cariprazine) as an Adjunctive Treatment for Major Depressive Disorder: May 28, 2019: Approval FDA Approves Expanded Use of Vraylar (cariprazine) in the Treatment of Bipolar Depression: Nov 13, 2017: Approval Allergan Receives FDA Approval for Vraylar (cariprazine) in the Maintenance Treatment of Schizophrenia

U.S. FDA Approves VRAYLAR® (cariprazine) as an Adjunctive Treatment for
VRAYLAR is an oral, once-daily atypical antipsychotic approved as an adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD) in adults (1.5 or 3 mg/day), for the treatment of depressive episodes associated with bipolar I disorder (bipolar depression) in adults (1.5 or 3 mg/day), and for the acute treatment of.

Cariprazine Approved by FDA as Adjunctive Treatment for MDD
AbbVie (NYSE: ABBV) today announced that the U.S. Food and Drug Administration (FDA) has approved VRAYLAR® (cariprazine) as an adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD) in adults. Supported by clinical data demonstrating efficacy and well-established tolerability, this additional indication provides a new option for adults who have a partial.

Vraylar (Cariprazine) for Schizophrenia
Cariprazine is marketed as Vraylar in the U.S., and in addition to being approved as an adjunctive therapy to antidepressants for the treatment of MDD in adults, it is FDA-approved to treat adults with depressive, acute manic and mixed episodes associated with bipolar I disorder, as well as schizophrenia. Cariprazine is co-developed by AbbVie.

U.S. FDA Approves VRAYLAR® (cariprazine) as an Adjunctive Treatment for
VRAYLAR is an oral, once-daily atypical antipsychotic approved as an adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD) in adults (1.5 or 3 mg/day), for the.