
(PDF) Poststreptococcal reactive arthritis what is it and how do we
The arthritis usually responds within three days to nonsteroidal anti-inflammatory drugs. Poststreptococcal reactive arthritis is nonmigratory, can affect any joint, and typically does not respond.
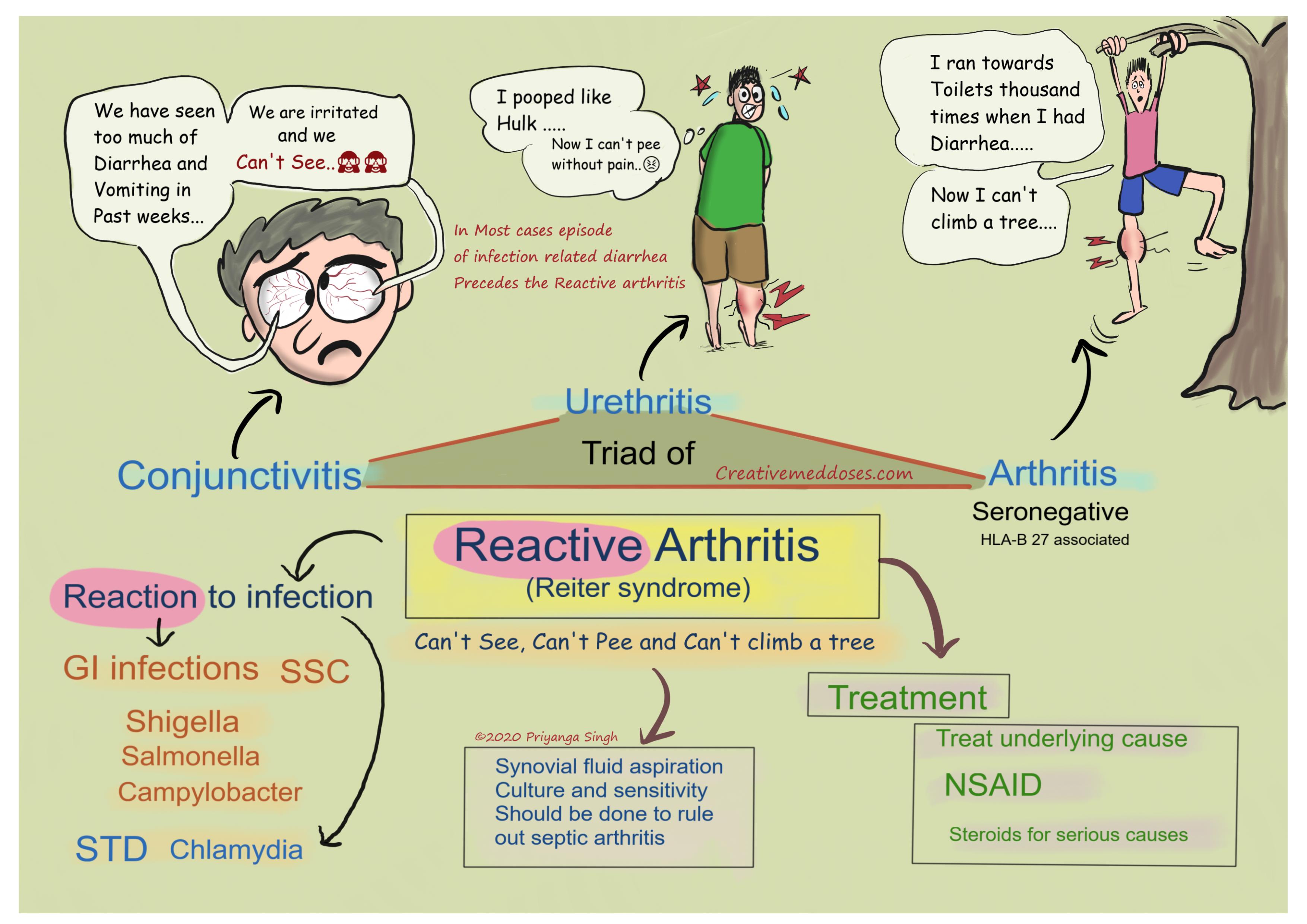
Reactive arthritis Quick review Creative Med Doses
Post-streptococcal reactive arthritis (PSRA) has now emerged as a different clinical entity to acute rheumatic fever. PSRA should be considered as one of the differentials for acute polyarthritis in adults. There is no agreement about the need and duration of penicillin prophylaxis for PSRA in current literature.

Poststreptococcal reactive arthritis in Japan Journal of Infection
Abstract. Poststreptococcal reactive arthritis (PSRA) is associated with prior group A β-hemolytic streptococcal infection and has a reported annual incidence of 1 to 2 cases per 100,000 persons, approximately twice that of acute rheumatic fever (ARF) in the US. Children who present with reactive arthritis are not uncommon in a busy general.
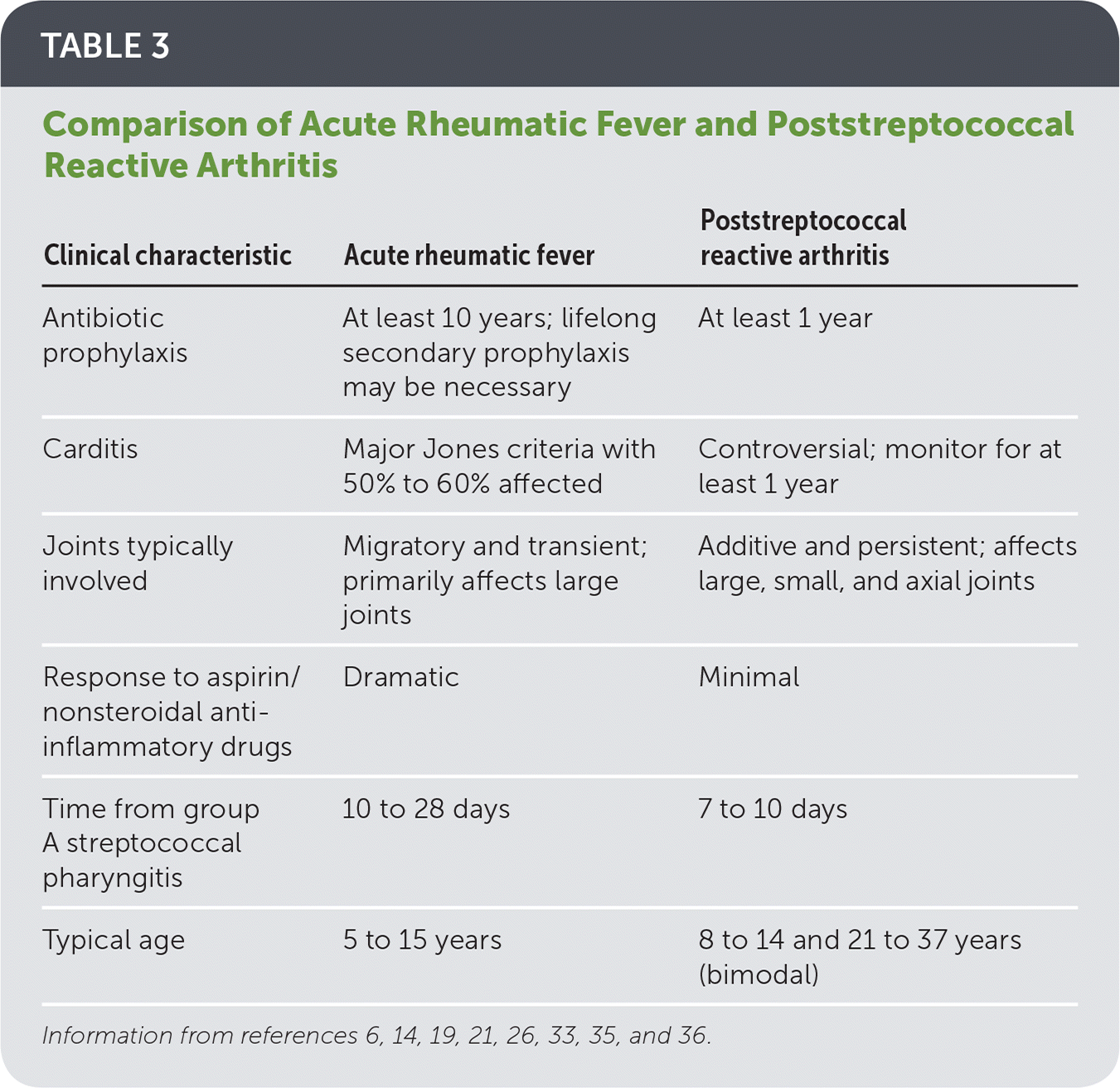
Poststreptococcal Illness Recognition and Management AAFP
Summary. PSRA is an inflammatory joint condition that can develop after a person recovers from strep throat. Aside from typical features of arthritis, other symptoms can include nodules in the.

PPT DIAGNOSIS & MANAGEMENT OF RHEUMATIC FEVER PowerPoint Presentation
Reactive arthritis is conventionally defined as an arthritis that arises following an infection, although the pathogens cannot be cultured from the affected joints. It is generally regarded as a form of spondyloarthritis (SpA). The definition, clinical features, approach to diagnosis and differential diagnosis, and management of reactive.

What is Reactive arthritis? Know the causes, symptoms, & Ayurvedic tips
Post-streptococcal reactive arthritis (PSRA) has now emerged as a different clinical entity to acute rheumatic fever. PSRA should be considered as one of the differentials for acute polyarthritis in adults. There is no agreement about the need and duration of penicillin prophylaxis for PSRA in current literature.

What is poststreptococcal reactive arthritis (PRSA)? An overview
In 1982 Goldsmith and would not be justified, we did not revise the published diagnoses Long described a poststreptococcal syndrome in children, char- other than to exclude patients with arthralgia but no arthritis, acterized by symmetrical arthritis followed by intense arthralgia although some authors [11, 12] acknowledged that some patients that was poorly responsive to aspirin therapy [5.

Poststreptococcal Reactive Arthritis Diagnostic Challenges The
8. Vol. 43. Oxford; 2004. Poststreptococcal reactive arthritis: what is it and how do we know? pp. 949-54. [Google Scholar] Simonini G, Taddio A, Cimaz R. No evidence yet to change American Heart Association recommendations for poststreptococcal reactive arthritis: comment on the article by van Bemmel et al. Arthritis Rheum.

Reactive Arthritis Knee
The arthritis associated with ARF is migratory, responds well to aspirin/NSAIDs and usually improves in 2-3 weeks. 6-8 In adults, reactive arthritis secondary to recent streptococcal infection is more likely to be missed as pharyngitis is not a common initial presentation of streptococcal infection. 6 7 9 The expressions of HLA DRB1*01 and HLA DRB1*16 alleles are increased in PSRA and ARF.
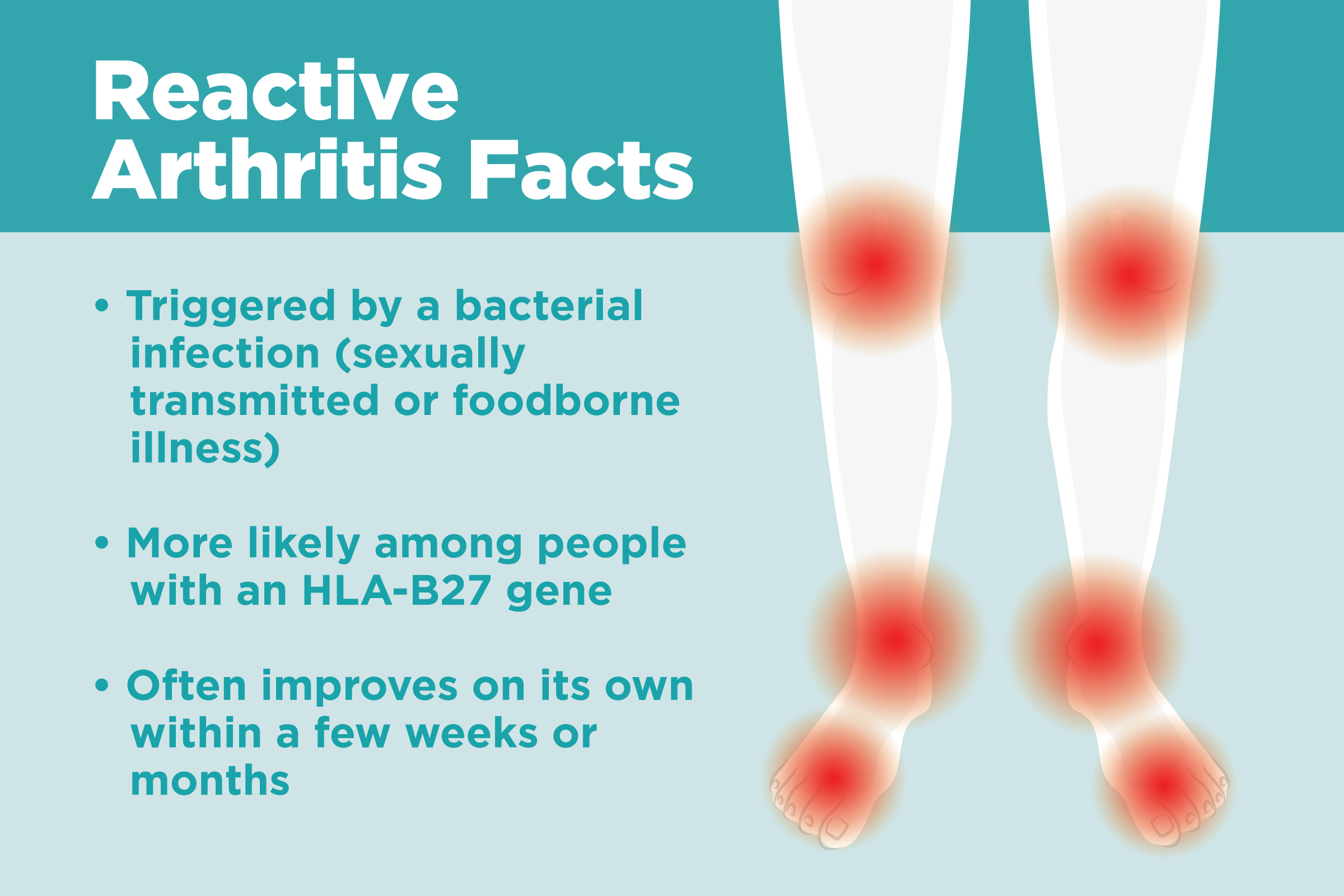
What Is Reactive Arthritis? Understanding Symptoms, Causes, and Treatment
Search worldwide, life-sciences literature Search. Advanced Search Coronavirus articles and preprints Search examples: "breast cancer" Smith J
(PDF) Poststreptococcal reactive arthritis in children A distinct
Poststreptococcal reactive arthritis is a type of sterile arthritis which occurs after a betahemolytic streptococcal throat or skin infection. It was defined as scarlatinal arthritis in 1959.

Реактивный Артрит Рентгенологические Признаки — Buyartox.ru
The name post-streptococcal 'reactive arthritis' may bring to mind the picture of classical reactive arthritis (ReA) that is a spondyloarthritis occurring after gut or urogenital infection. However, classical ReA, unlike PSRA, has a preponderance for lower limb large joints, affects the sacroiliac joint and axial skeleton, and is associated with HLA-B27 in a large majority [ 13 ].

Hypothesized model of pathogenesis in poststreptococcal reactive
Introduction: Post-Streptococcal Reactive Arthritis (PSRA) is defined as inflammatory arthritis of ≥1 joint associated with a recent group A streptococcal infection in a patient who does not fulfill the Jones criteria for the diagnosis of Acute Rheumatic Fever (ARF). Methods: In this narrative review, we conducted a systematic search on MEDLINE, EMBASE, Cochrane Library and Google Scholar.
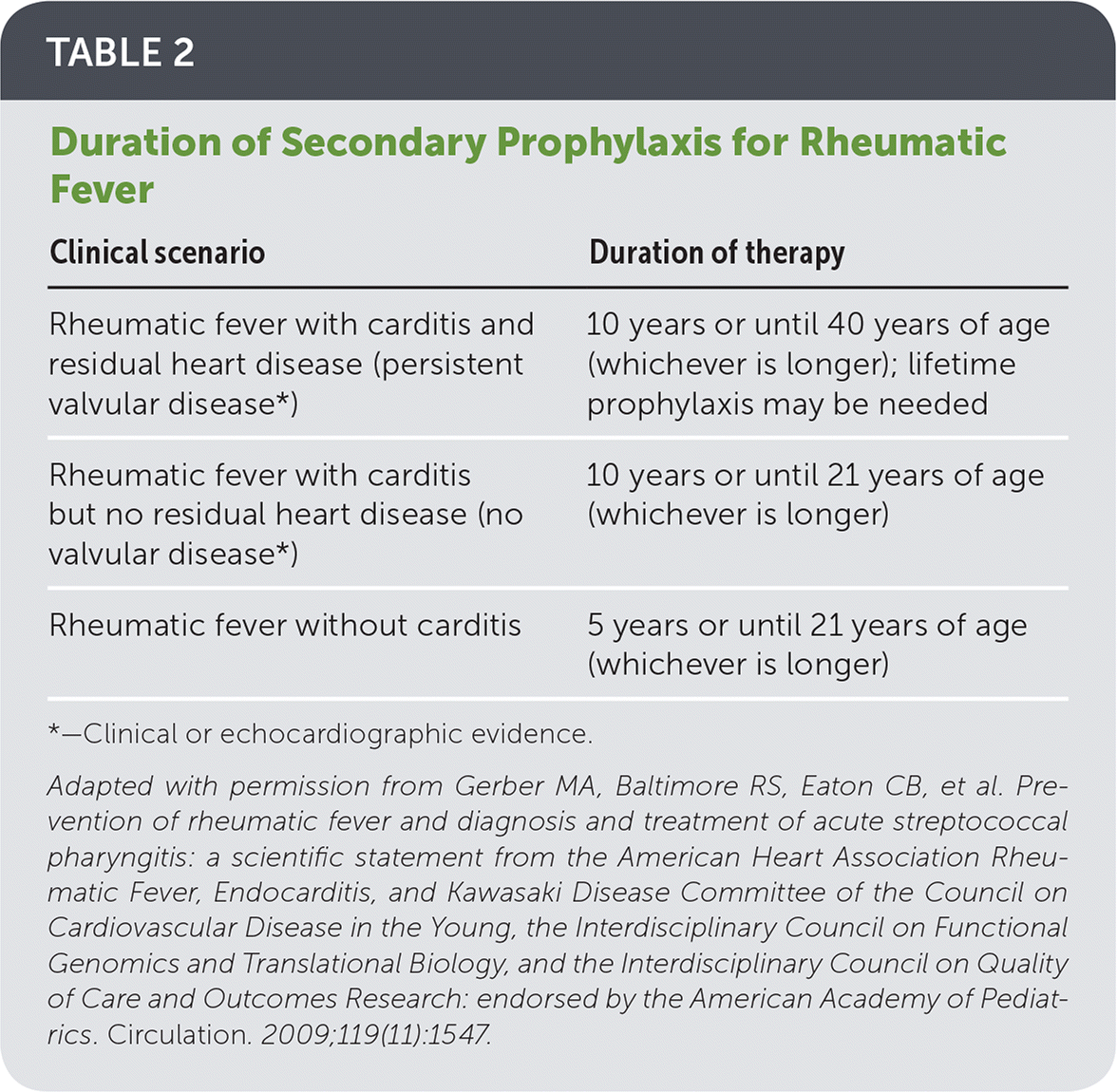
Poststreptococcal Illness Recognition and Management AAFP
Poststreptococcal reactive arthritis (PSRA) is associated with prior group A β-hemolytic streptococcal (GABHS) infection and has a reported annual incidence of 1 to 2 cases per 100,000 persons,1 approximately twice that of acute rheumatic fever (ARF) in the US.2 In comparison, the incidence of reactive arthritis from bacterial enteric infections is 0.6 to 3.1 cases per 100,000 persons in.

Post Streptococcal Reactive Arthritis YouTube
Poststreptococcal reactive arthritis has been proposed as a homogeneous clinical entity distinct from acute rheumatic fever and from other forms of reactive arthritis. The primary aim of this paper is to consider whether the available data provide a clear or reasonable foundation for this concept; the pooling of the case reports in this analysis should not be taken to imply an assumption that.

Hypothesized model of pathogenesis in poststreptococcal reactive
Objective: To find out whether poststreptococcal reactive arthritis (PSRA) is a discrete, homogeneous clinical syndrome. Method: Literature review from case reports and case series. Results: One hundred and eighty-eight cases were identified. The age distribution was bimodal, with one peak in childhood and one peak in adulthood.