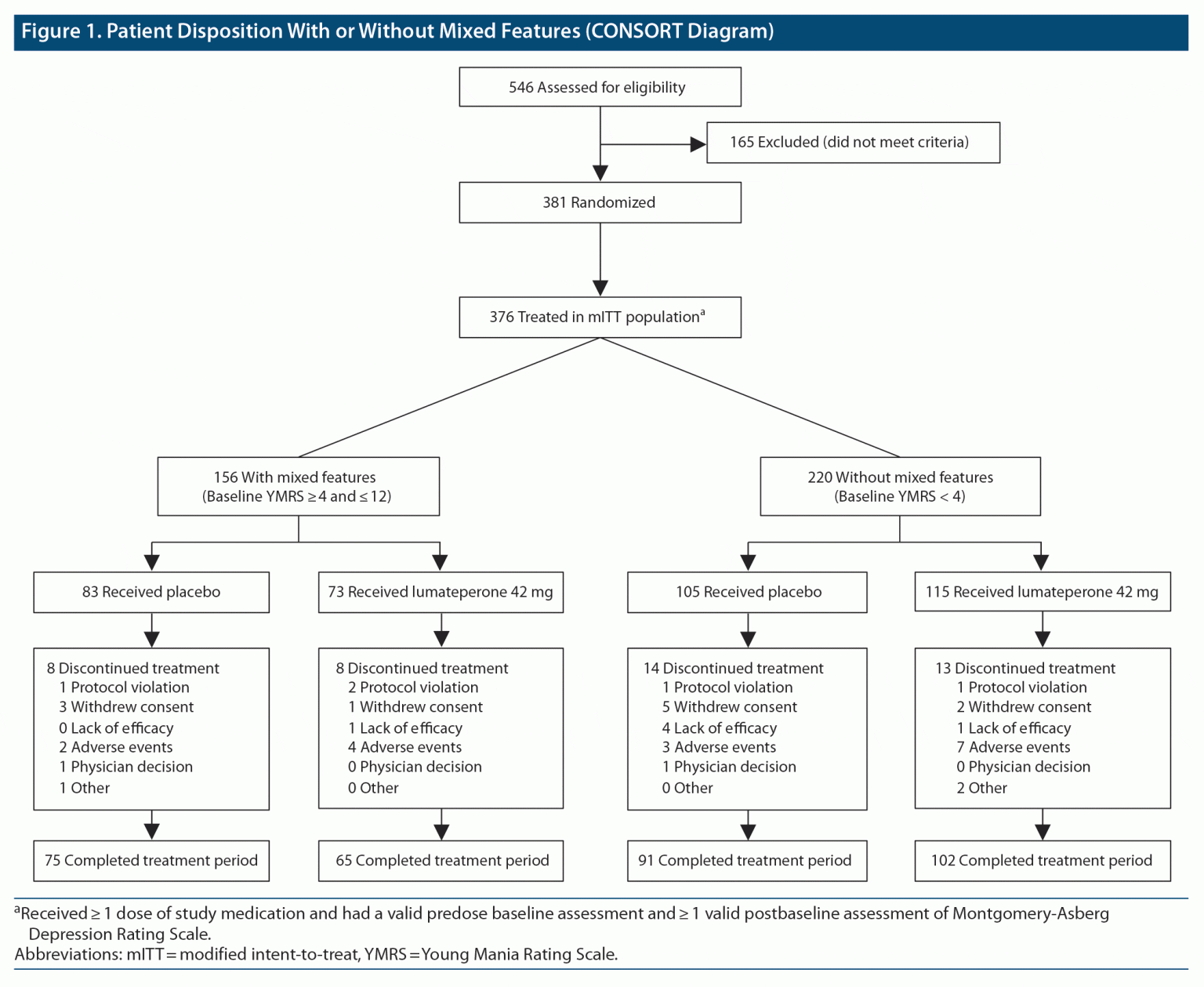
Efficacy of Lumateperone in Bipolar Depression With Mixed Features
Lumateperone 42 mg significantly improved symptoms of depression and disease severity in patients with an MDE associated with bipolar I or bipolar II disorder, with or without mixed features. Objective: A post hoc analysis of a phase 3, randomized, double-blind, placebo-controlled outpatient study investigated efficacy of lumateperone 42 mg in patients with bipolar I or bipolar II disorder and.

Cariprazine Shows Efficacy for Bipolar Mania With Mixed Features
Objective: In a phase 3 randomized double-blind placebo-controlled study, the authors investigated the efficacy and safety of 42 mg/day of lumateperone in patients with bipolar I or bipolar II disorder experiencing a major depressive episode. Methods: Patients 18-75 years old with a clinical diagnosis of bipolar I or bipolar II disorder and experiencing a major depressive episode were.
Combine these screening tools to detect bipolar depression MDedge
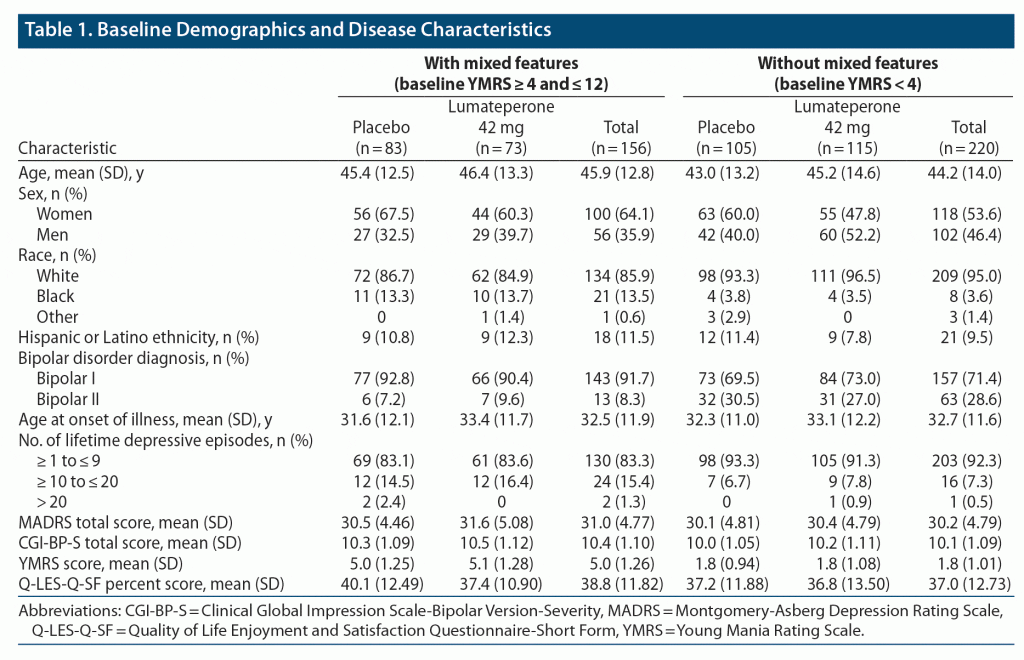
According to the DSM-IV criteria, 58 (24.5%) were diagnosed with MDD and 73 (30.8%) were diagnosed with bipolar depression. Bipolar depression with mixed features was diagnosed in 106 (44.7%.
Efficacy and Safety of Lumateperone for Major Depressive Episodes
The reported prevalence of MDEs with mixed features has ranged from 46.4% to 73.1% in bipolar disorder (BD) and from 7.6% to 48.7% in major depressive disorder (MDD).1-6) Mixed features are associated with a more severe clinical course, including earlier onset, frequent recurrence, greater risk of comorbid disorders, longer time to remission, poorer response to pharmacological treatment, and.
Efficacy of Lumateperone in Bipolar Depression With Mixed Features
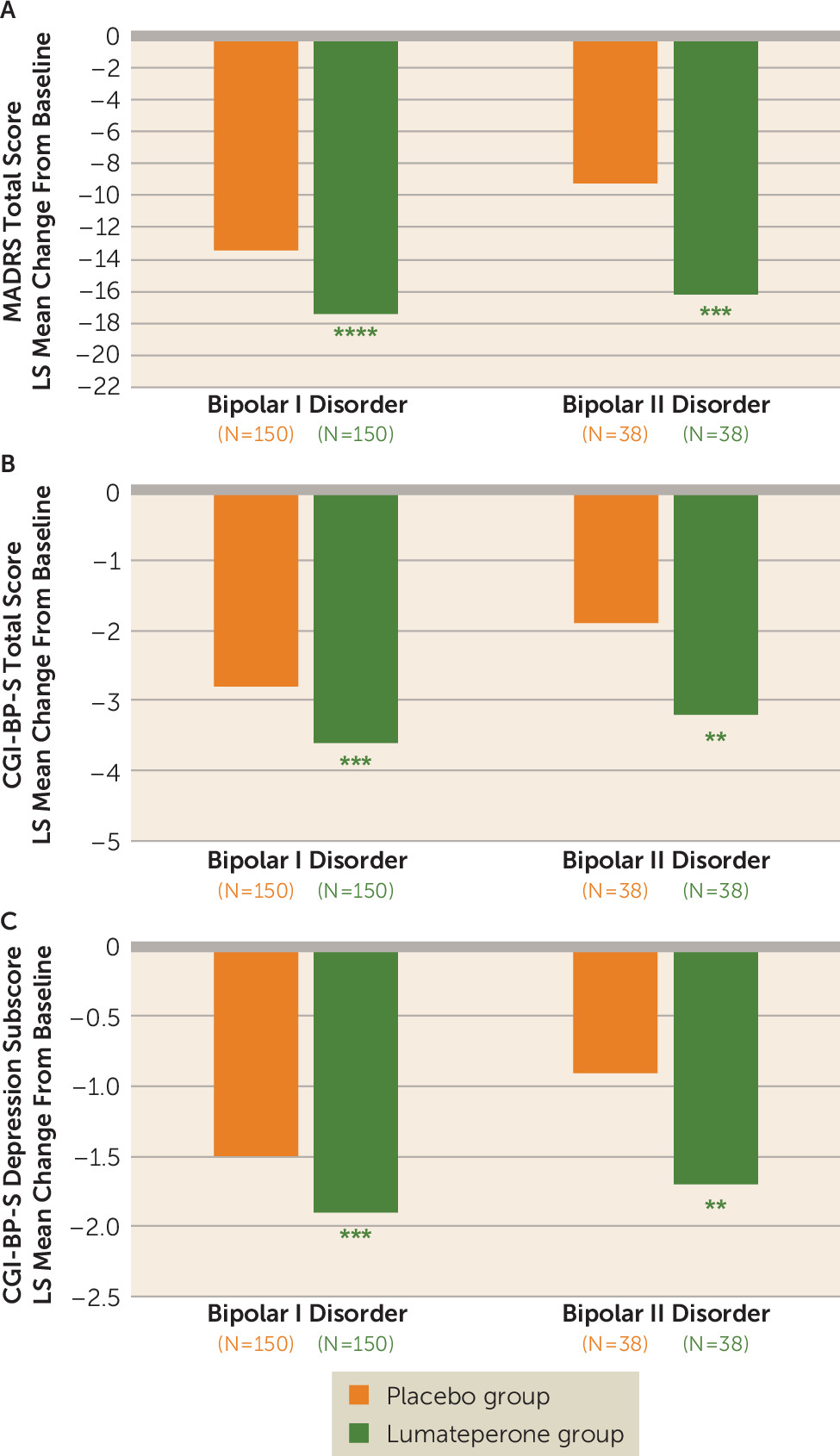
Additionally, lumateperone treatment significantly improved quality of life as measured by Q-LES-Q-SF percent score in patients with mixed features. The latter improvement is notable because, compared with other bipolar disorder states, patients experiencing mixed features have reported reduced quality of life across several domains. 3.
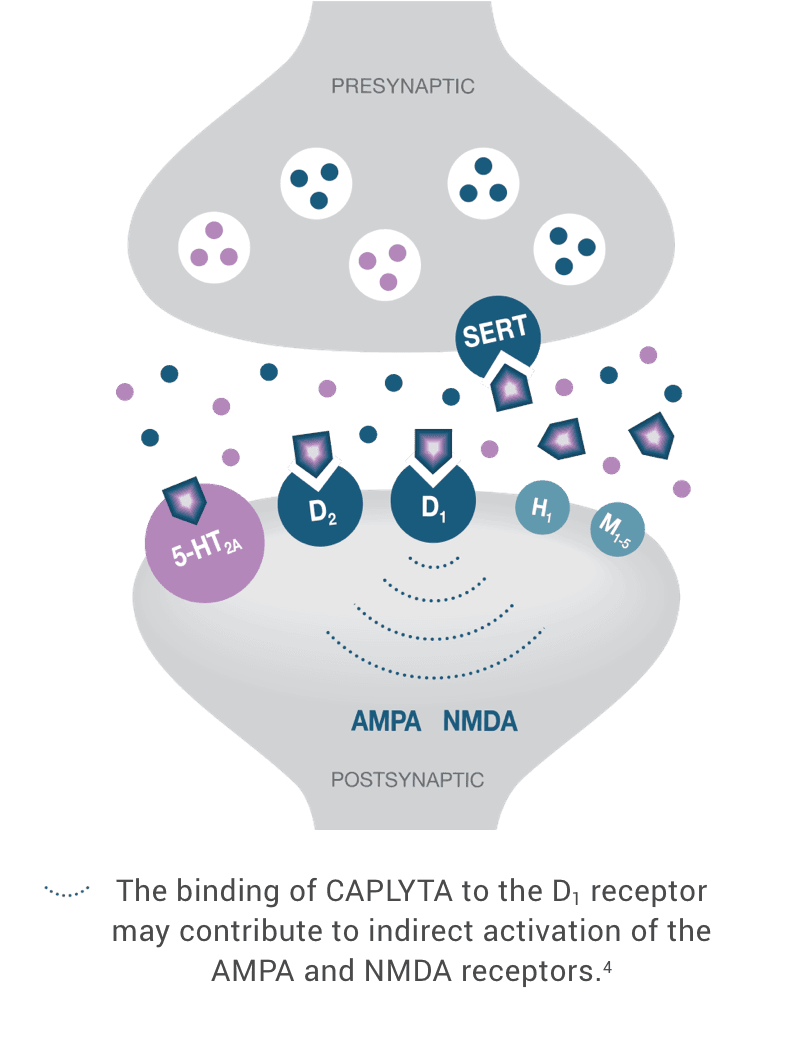
Pharmacology in Bipolar Depression CAPLYTA® (lumateperone)
Study 403 was a randomized, double-blind, placebo-controlled, global study to evaluate the efficacy and safety of lumateperone as monotherapy treatment for patients with major depressive episodes associated with MDD or Bipolar I or Bipolar II Disorder who also met the Diagnostic and Statistical Manual of Mental Disorder, 5 th Edition (DSM-5) criteria for mixed-features. Additionally, patients.

Major Depressive Disorder with Mixed Features A Review
Lumateperone was recently approved as monotherapy or adjunctive therapy with lithium or valproate for treatment of depression in the context of either BD-I or BD-II disorder. 19 Lumateperone is unique in that it modulates serotonin, dopamine and glutamate simultaneously. 5 In a study of 377 patients, lumateperone had significantly greater MADRS response rate (51.1% vs 36.7%; OR=2.98, p<0.001.
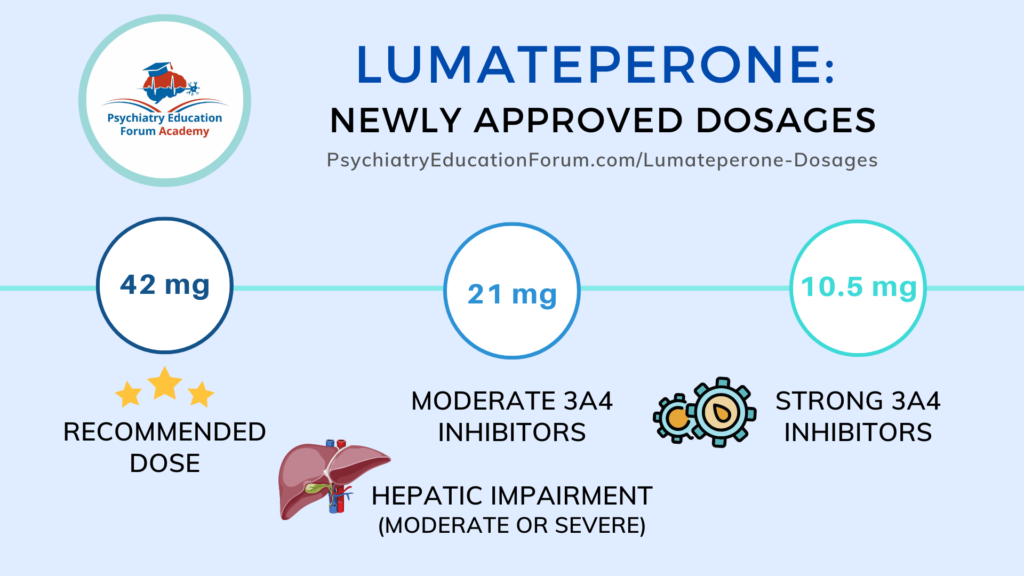
Lumateperone Newest FDA Approved Medication for Bipolar Depression
Recent results from study 403 showed that lumateperone (Caplyta) 42 mg monotherapy helped significantly reduce the severity of depressive symptoms in patients with mixed features in major depressive disorder (MDD) and in patients with mixed features in bipolar depression. "In this study, lumateperone demonstrated a robust effect in both patients with MDD with mixed features and patients with.

Bipolar with Mixed Features Causes, Symptoms and Treatment Drlogy
This is, to our knowledge, the largest network meta-analysis of pharmacotherapy for bipolar depression to date. Olanzapine plus fluoxetine, quetiapine, olanzapine, lurasidone, lumateperone, cariprazine, and lamotrigine were found to be more efficacious than placebo in adults with acute bipolar depression, with good confidence in the evidence, and to differ in their side-effect profiles. These.
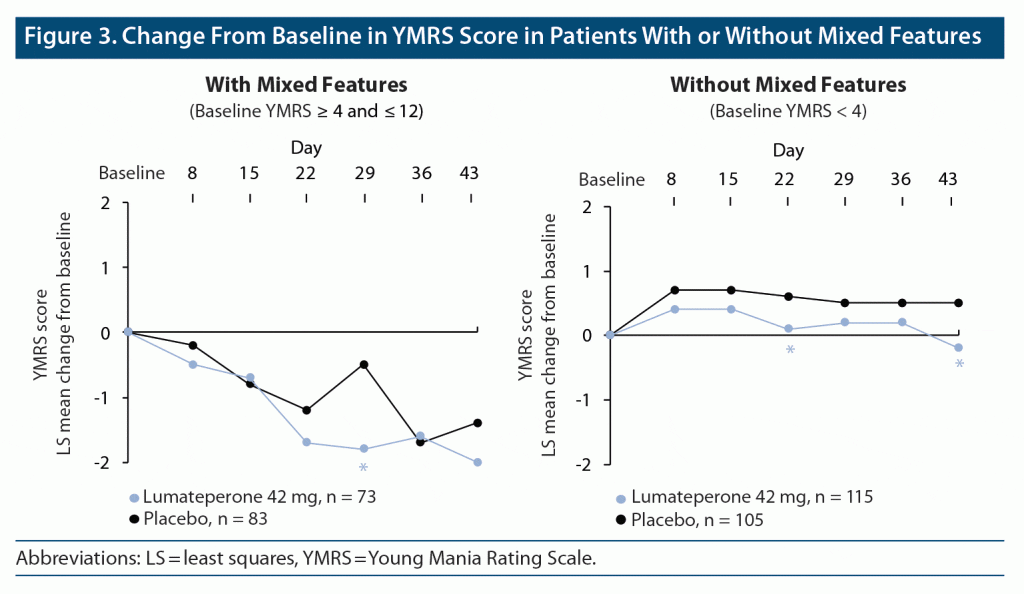
Efficacy of Lumateperone in Bipolar Depression With Mixed Features
Objective: In a phase 3 randomized double-blind placebo-controlled study, the authors investigated the efficacy and safety of 42 mg/day of lumateperone in patients with bipolar I or bipolar II disorder experiencing a major depressive episode. Methods: Patients 18-75 years old with a clinical diagnosis of bipolar I or bipolar II disorder and experiencing a major depressive episode were eligible.
:%0A%0ALumateperone for Depression, Bipolar Disorder.png?md=1)
Lumateperone for Depression, Bipolar Disorder Clinical Trial 2024 Power
Lumateperone was found, in a phase 3, randomized, double-blind, placebo-controlled trial, to improve depressive symptoms among patients with bipolar I and II disorders (BD) with major depressive.
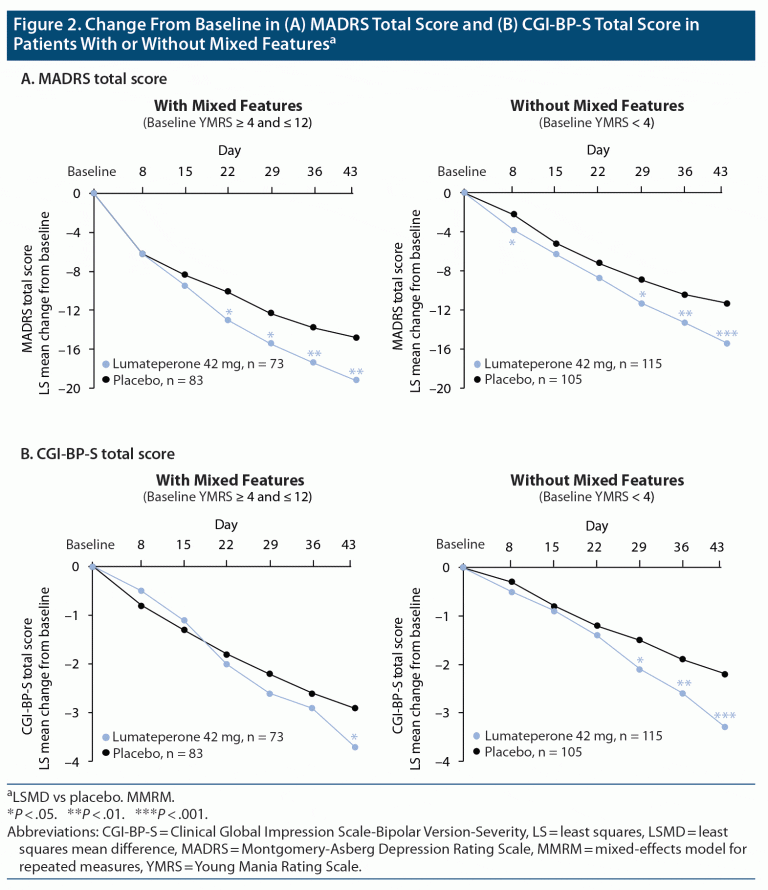
Efficacy of Lumateperone in Bipolar Depression With Mixed Features
Objective: This phase 3, randomized, double-blind, placebo-controlled study (NCT02600507) evaluated the efficacy and safety of lumateperone adjunctive therapy to lithium or valproate in patients with bipolar depression. Methods: Patients (18-75 years) with bipolar I or bipolar II disorder experiencing a major depressive episode (MDE), with inadequate therapeutic response to lithium or.

Lumateperone Newest FDA Approved Medication for Bipolar Depression
Lumateperone (lumateperone tosylate, ITI-007) is a mechanistically novel antipsychotic that is FDA approved for the treatment of schizophrenia in adults and for depressive episodes associated with bipolar I or bipolar II disorder (bipolar depression) in adults as monotherapy and as adjunctive therapy with lithium or valproate (Caplyta et al., 2022).

Lumateperone Newest FDA Approved Medication for Bipolar Depression
(DOI: 10.4088/jcp.22m14739) Objective: A post hoc analysis of a phase 3, randomized, double-blind, placebo-controlled outpatient study investigated efficacy of lumateperone 42 mg in patients with bipolar I or bipolar II disorder and experiencing a major depressive episode (MDE) stratified by the presence of mixed features. Methods: Adults (18-75 years) with bipolar I or bipolar II disorder.
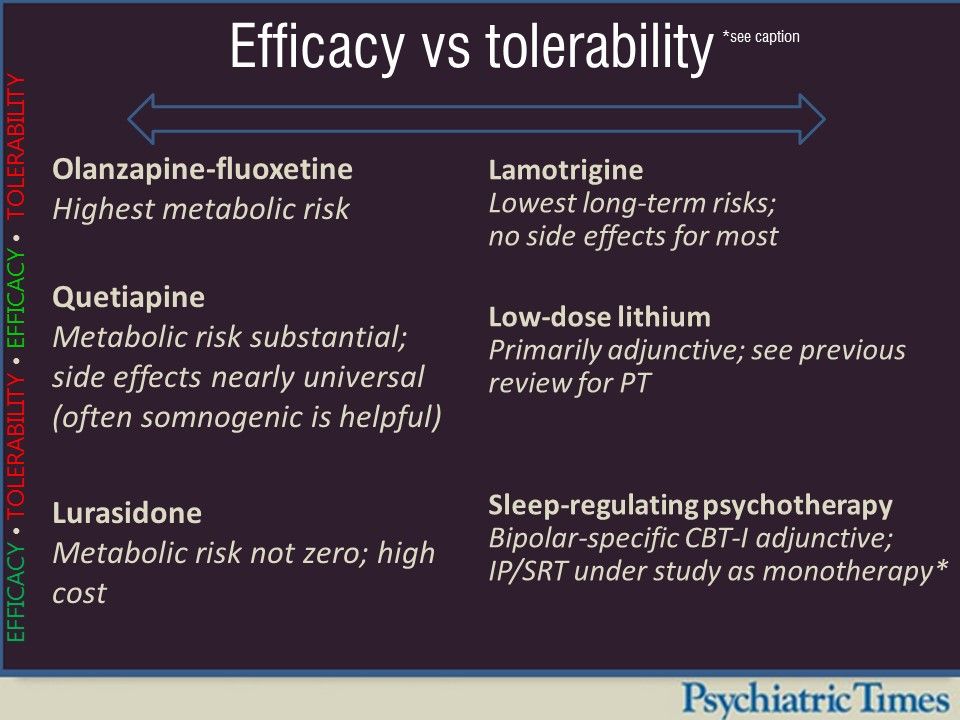
Efficacy Vs Tolerability in Bipolar Depression Psychiatric Times
March 28, 2023. Lumateperone monotherapy was associated with significant improvement of major depressive episodes in patients with major depressive disorder (MDD) with mixed features and in.
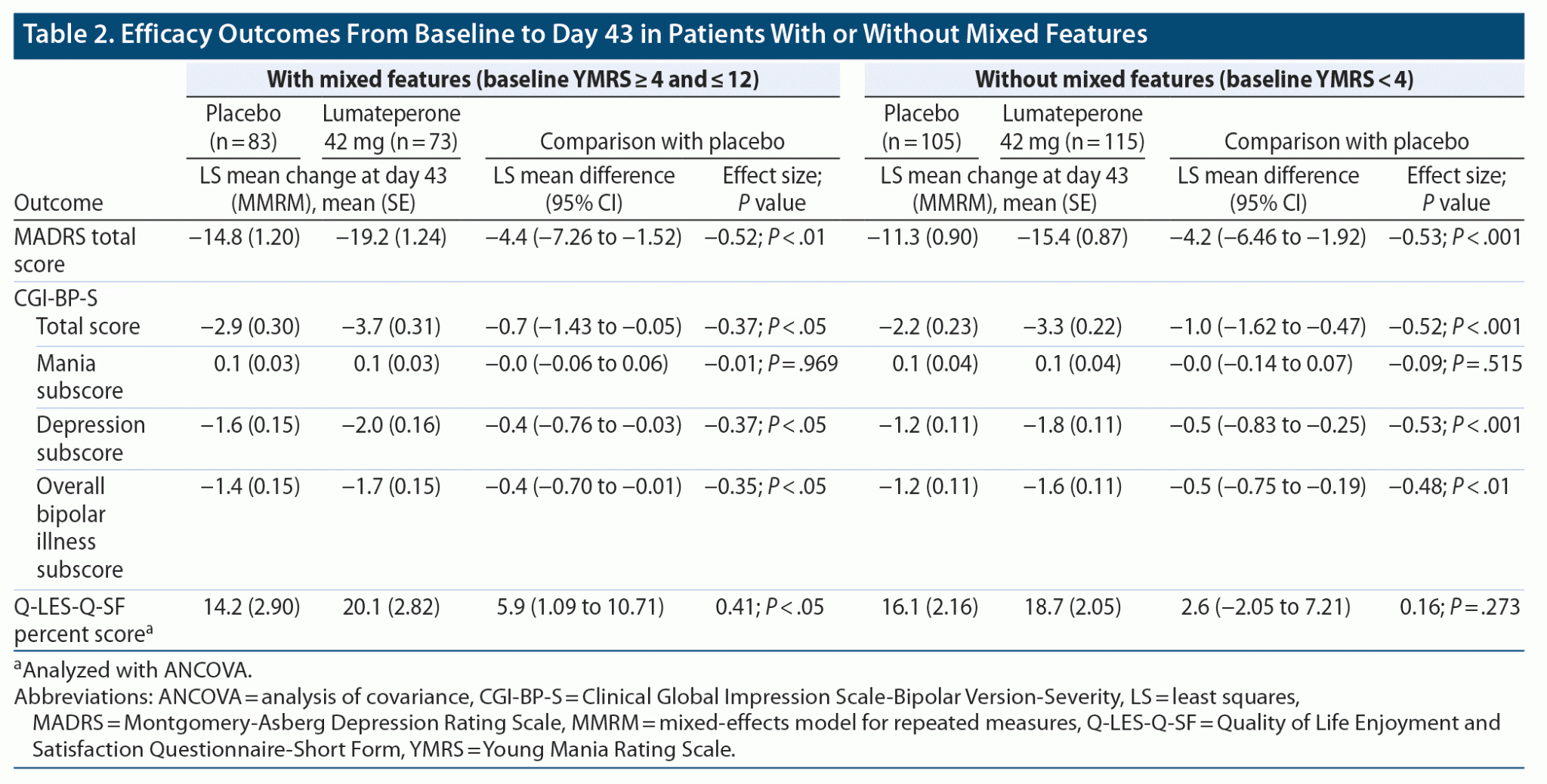
Efficacy of Lumateperone in Bipolar Depression With Mixed Features
Objective: A post hoc analysis of a phase 3, randomized, double-blind, placebo-controlled outpatient study investigated efficacy of lumateperone 42 mg in patients with bipolar I or bipolar II disorder and experiencing a major depressive episode (MDE) stratified by the presence of mixed features. Methods: Adults (18-75 years) with bipolar I or bipolar II disorder experiencing an MDE, defined by.